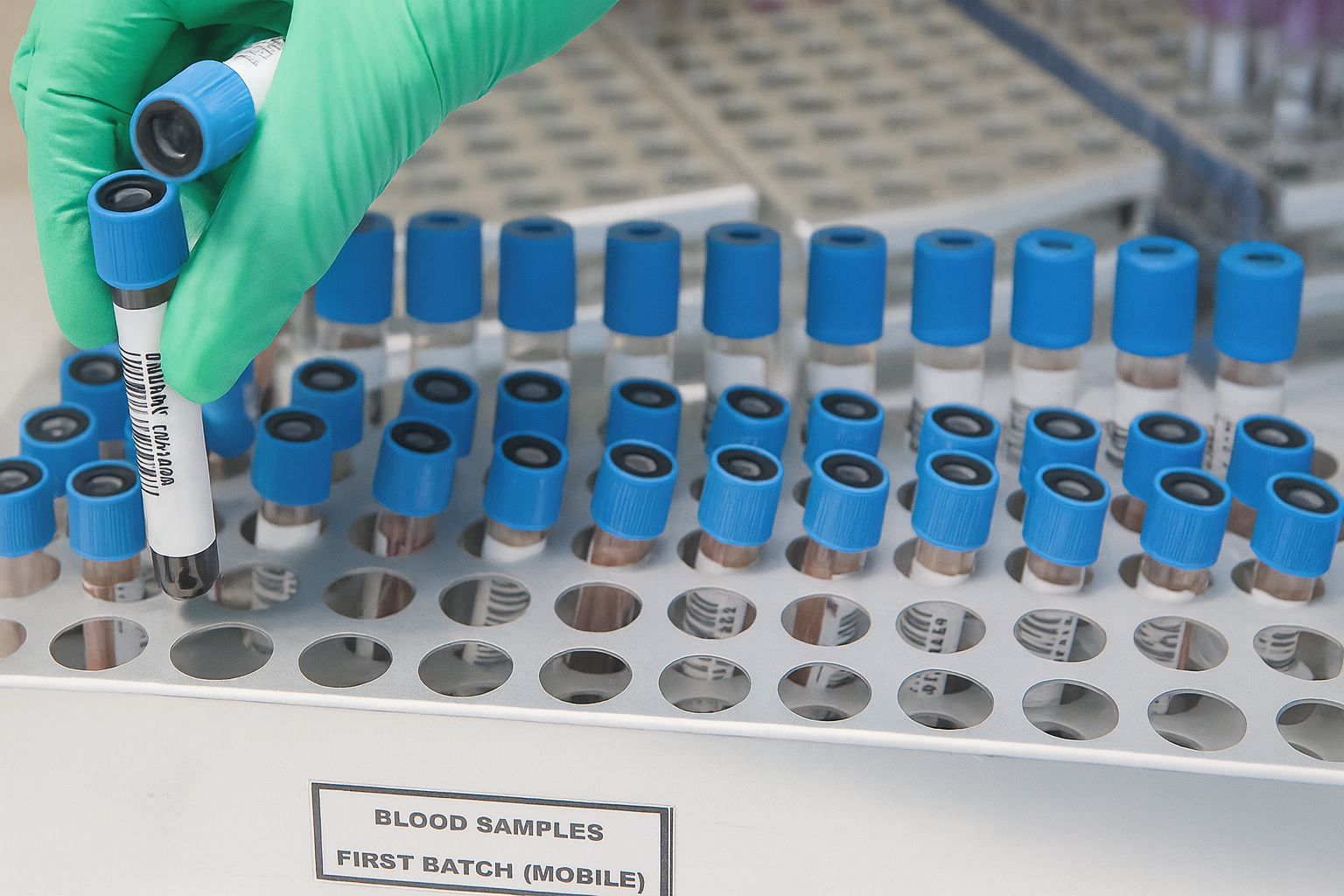
- Developed in Rzeszów: A Polish team (Fryderyk Chopin Medical University, Rzeszów) spent 2+ years creating a novel CLL test named “TAG-CLL” rmf24.pl. It was led by Dr. Marzena Wojtaszewska of Rzeszów’s University Clinical Hospital.
- IGHV gene sequencing: The TAG-CLL test reads patients’ immunoglobulin heavy-chain variable (IGHV) gene sequences to detect mutations rmf24.pl. Mutated vs. unmutated IGHV status is a well-known predictor of CLL aggressiveness cllsociety.org.
- Faster, cheaper testing: TAG-CLL cuts turnaround time from about 4 weeks to only ~1–2 weeks, and reduces cost by ~20% compared to conventional IGHV tests rmf24.pl.
- Validated and published: The new method was nationally validated – 12 molecular labs used standardized DNA samples and all amplicons were run through the unified TAG-CLL workflow pubmed.ncbi.nlm.nih.gov. Results appeared in the Journal of Molecular Diagnostics (Sept 2025) rmf24.pl, pubmed.ncbi.nlm.nih.gov.
- Expert backing: Polish hematology leaders (e.g. Prof. Mirosław Markiewicz) and international CLL experts (e.g. Mayo Clinic’s Dr. Curtis Hanson) praise the innovation. Hanson notes IGHV mutation testing must be “top of mind” for CLL prognosis captodayonline.com, and Markiewicz says the test “quickly yields information allowing modern, safe and effective therapy” rmf24.pl.
- Patient impact: By speeding up risk classification, TAG-CLL can help doctors start targeted treatments sooner. As Dr. Talha Munir (St. James’s Hospital, UK) emphasizes, “IGHV mutation status is one of the most important predictors of how much time will pass before the first treatment is needed in CLL” cllsociety.org – meaning earlier answers could translate to better-tailored care.
Revolutionary New CLL Test from Rzeszów
Researchers at the University Clinical Hospital in Rzeszów (affiliated with Fryderyk Chopin University of Medical Sciences) have announced a groundbreaking test for chronic lymphocytic leukemia (CLL). CLL is the most common adult leukemia, and its course can range from very slow-growing to aggressive. A key molecular marker is the “somatic hypermutation” status of the patient’s IGHV immunoglobulin genes: mutated IGHV generally means a milder, indolent disease, while unmutated IGHV signals a more aggressive form cllsociety.org. However, standard IGHV testing has long been slow and costly. The new TAG-CLL test (TAgmentation-based Genotyping for CLL) promises to change that.
In practice, TAG-CLL works by taking a blood or bone marrow sample, amplifying the relevant IGHV gene fragments, and then using a clever “tagmentation” process to prepare them for sequencing. Tagmentation fragments the DNA and adds sequencing tags in one step, allowing labs to run the material on either Sanger sequencing or next-generation sequencers under a common protocol pubmed.ncbi.nlm.nih.gov. This streamlined pipeline means that TAG-CLL can directly compare the DNA libraries from different machines, overcoming previous inconsistencies. As the team’s Journal of Molecular Diagnostics paper explains, TAG-CLL is “agnostic to the amplification protocol used,” unifying results across platforms pubmed.ncbi.nlm.nih.gov. In tests, all 12 collaborating labs amplified a standard mixture of IGHV genes and then ran the products through the TAG-CLL workflow – achieving consistent mutation reads and germline identity measures from lab to lab pubmed.ncbi.nlm.nih.gov.
“The TAG-CLL method is used to determine the aggressiveness of leukemia,” explains Dr. Marzena Wojtaszewska, lead inventor of the test rmf24.pl. In her words, “using the test we read the immunoglobulin gene sequence and infer whether the gene is mutated or not. From this it is possible to determine the patient’s prognosis.” In short, TAG-CLL reads the exact IGHV sequence and checks the mutation load; this single test replaces older methods that required time-consuming Sanger runs or expensive NGS panels. According to the developers, TAG-CLL achieves the same diagnostic endpoint much faster and more cheaply than before rmf24.pl.
How TAG-CLL Beats Current Standards
Until now, most labs had two choices for IGHV analysis: Sanger sequencing, which is reliable but involves multiple PCRs and “walking” sequencing reactions (often taking weeks), or next-generation sequencing (NGS) panels, which can be faster but require complex bioinformatics and expensive equipment. Both approaches have drawbacks. As the research paper notes, IGHV mutation testing “poses serious methodological problems” for laboratories: Sanger is slow and labor-intensive, while NGS, although powerful, is costly and was not standardized for IGHV pubmed.ncbi.nlm.nih.gov. Small differences in how each lab runs the PCR can lead to inconsistent results between centers.
TAG-CLL is designed to overcome these issues. By performing a universal tagmentation step after the PCR, every lab’s sample is put into a common sequencing format. This means that a single analysis pipeline can detect IGHV mutations even if the raw amplification was done slightly differently. In their multi-lab trial, the Rzeszów team found that TAG-CLL yielded highly concordant results: the same IGHV clones were detected, and mutation percentages matched across labs. In effect, TAG-CLL “reveals the weak points of both Sanger and NGS,” as the paper subtitle puts it, by providing a cross-platform check on accuracy pubmed.ncbi.nlm.nih.gov.
In practical terms, the new method cuts turnaround time dramatically. Until now, patients waited about a month for standard IGHV results. “TAG-CLL shortens the wait from a month to two weeks,” the team reports rmf24.pl. Moreover, by automating the library prep, it lowers reagent and labor costs. The hospital press release notes that TAG-CLL is about 20% cheaper than existing tests rmf24.pl. In Polish hospitals this means saving hundreds of euros per patient – an important factor in public health settings.
Promising Validation and Publications
The TAG-CLL test was developed through close collaboration between the Rzeszów University Hospital hematology clinic, its molecular biology lab, and the University of Rzeszów medical faculty. After laboratory development, the method was formally validated: researchers prepared an “oligoclonal” DNA sample (mixing known IGHV gene fragments) and asked 12 reference labs across Poland to run their usual amplifications. All the resulting products were then sequenced using TAG-CLL. The published results show high concordance in productive clonotypes (ie. the main leukemia sequences) and matching “germline identity” percentages of mutation pubmed.ncbi.nlm.nih.gov.
Results of these trials were published online in The Journal of Molecular Diagnostics in September 2025 rmf24.pl, pubmed.ncbi.nlm.nih.gov. (The JMD paper, led by Wojtaszewska et al., details the protocol and comparisons of Sanger vs. NGS vs. TAG-CLL.) According to the hospital, the method and validation data have also been shared at workshops – already three editions in 2024–2025 – training geneticists from major oncology centers (Kraków, Warsaw, etc.) on how to run TAG-CLL. Following these workshops, several other laboratories have started using the technique, demonstrating its practical rollout rmf24.pl.
Expert Reactions
Polish and international experts are enthusiastic. Prof. Mirosław Markiewicz, head of Hematology at Rzeszów’s University Hospital, co-authored the work. He emphasizes the patient benefit: “Chronic lymphocytic leukemia is the most common blood cancer in adults. Thanks to the new method implemented at our hospital, we quickly obtain information that allows [us] to apply modern, safe and effective therapy for patients,” he says rmf24.pl. In other words, faster IGHV results mean doctors can start targeted therapies sooner or avoid unnecessary chemo – exactly the kind of precision oncology that improves outcomes.
International voices echo the significance of IGHV testing. In the U.S., Dr. Curtis Hanson of the Mayo Clinic underscores that “IGHV mutation analysis should be top of mind when acquiring prognostic and potentially therapeutic information in chronic lymphocytic leukemia.” captodayonline.com Formerly described in a CAP Today interview, his quote highlights that knowing IGHV mutation status is critical for determining prognosis and therapy choice. Similarly, Dr. Talha Munir of St. James’s University Hospital (Leeds, UK) notes that IGHV status predicts disease course: “IGHV mutation status is one of the most important predictors of how much time will pass before the first treatment is needed,” he explains cllsociety.org. These experts did not develop TAG-CLL, but their comments stress why a faster, reliable IGHV test is a game-changer in CLL care.
The hospital’s director, Marcin Rusiniak, even hailed the breakthrough as a sign that Rzeszów is becoming a leader in cancer diagnostics: “Development of modern genetic diagnostics is one of our priorities. Achievements like this prove that Rzeszów is on the right path to becoming a leader in molecular diagnostics for oncology patients,” he said rmf24.pl.
Implications for Patients
For CLL patients, the TAG-CLL test could mean quicker, more personalized care. Currently, oncologists often know a patient has CLL (by blood tests or a biopsy) but then wait on detailed IGHV mutation results before finalizing the treatment plan. With TAG-CLL cutting lab processing time in half, that wait shortens considerably. In practice, this can allow high-risk (unmutated IGHV) patients to be started sooner on newer targeted drugs (like BTK inhibitors) rather than waiting for results or receiving a one-size-fits-all therapy. Lower testing costs may also allow more routine use of IGHV testing, bringing advanced diagnostics to smaller clinics and even earlier disease stages.
Importantly, TAG-CLL does not replace other CLL tests; it complements the standard flow cytometry and FISH panels. It specifically expedites a molecular test that had been lagging. As Dr. Markiewicz puts it, the method helps “quickly obtain information” for modern therapy, underscoring that diagnostics and treatment are increasingly linked rmf24.pl.
While TAG-CLL itself is a diagnostic tool rather than a treatment, its impact is measured in patient outcomes. Faster risk stratification can translate into reducing “watch and wait” periods or choosing the right treatment sooner. In the words of Dr. Munir, knowing IGHV mutation status helps decide “how much time will pass before first treatment is needed” cllsociety.org – an essentially prognostic question. By answering it faster and more affordably, TAG-CLL represents a significant advance in CLL management.
Sources: Announcement and interviews from Rzeszów University Hospital rmf24.pl; Jadczyk et al., Journal of Molecular Diagnostics (2025) pubmed.ncbi.nlm.nih.gov; expert commentaries captodayonline.com, cllsociety.org.